Introduction
Decomposition reactions, though less commonly discussed than their more glamorous counterparts like combustion or synthesis, play a pivotal role in chemistry. At their core, they involve a single compound breaking down into two or more simpler substances. These reactions occur in various settings, from natural processes within the environment to deliberate inductions in laboratories. But what drives this “chemical breakup,” and why is it essential to understand? Let’s dive into the heart of decomposition reactions.
What is a Decomposition Reaction? Unraveling the Basics
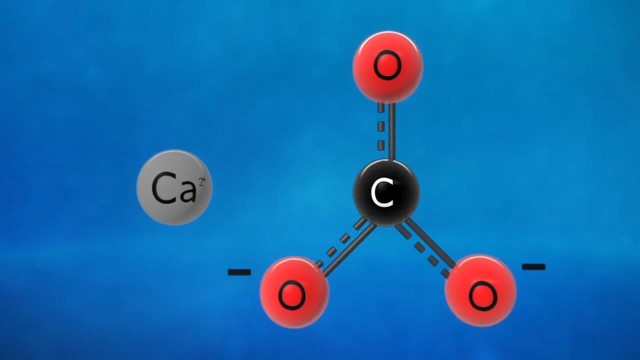
Simply put, a decomposition reaction occurs when one reactant breaks down to form two or more products. Generally induced by Heat, light, or electricity, the response can be represented as AB → A + B. In this formula, AB, a compound, decomposes to yield two separate elements or simpler compounds, A and B. Common examples include the breakdown of water into hydrogen and oxygen or the decomposition of calcium carbonate to calcium oxide and carbon dioxide.
The Energy Factor: Understanding Endothermic Reactions
Most decomposition reactions are endothermic. This means they absorb energy from their surroundings, typically in the form of Heat. Take the example of the decomposition of calcium carbonate: CaCO₃ (s) → CaO (s) + CO₂ (g) + Heat. In this reaction, calcium carbonate absorbs energy to break down into calcium oxide and carbon dioxide. Such endothermic nature ensures that decomposition reactions, especially in a lab setting, require a continuous energy supply.
Electrolytic Decomposition: Powering Breakdown with Electricity
Not all decomposition reactions are driven by Heat. Some are propelled by electricity in a process known as electrolysis. Electrolytic decomposition involves breaking down a compound by passing an electric current through it. A classic example is the electrolysis of water, where water decomposes into hydrogen and oxygen gases when an electric current is passed through it. This method showcases the versatility of decomposition reactions, emphasizing that various energy sources can initiate the breakdown process.
Significance in Everyday Life: Decomposition Around Us
Decomposition reactions aren’t limited to lab settings; they happen everywhere. For instance:
- Photodecomposition: Some compounds decompose when exposed to light. An everyday example is the breakdown of silver chloride to silver and chlorine upon light exposure.
- Biological Decomposition: In nature, decomposition reactions are integral to processes like respiration. Glucose decomposes to provide energy, water, and carbon dioxide, vital for cellular functions.
Challenges and Limitations: Not All Breakdowns Are Simple
While decomposition reactions might seem straightforward, they come with challenges. Not all compounds decompose quickly. Some require incredibly high temperatures or specific catalysts to initiate the breakdown. Additionally, ensuring the purity of the resulting products can be tricky, especially if multiple decomposition pathways are possible.
Decomposition in Industry: Practical Applications
The industrial world harnesses the power of decomposition reactions in various applications:
- Metal Extraction: Many metals are extracted from their ores using decomposition reactions. For instance, bauxite, an aluminum ore, undergoes decomposition to yield pure aluminum.
- Production of Gases: Many industrial processes require pure gases, which can be obtained through the corruption of compounds. For instance, decomposing water yields hydrogen, a key player in the fuel industry.
Safety Protocols in Decomposition Reactions
Safety first is a mantra that scientists and researchers live by. Adopting strict safety protocols is paramount, given the unpredictable nature of specific decomposition reaction. Protective equipment like lab coats, safety goggles, and gloves are non-negotiable in laboratory settings. Additionally, fume hoods are often employed when gases are expected as products to ensure proper ventilation and prevent any harmful inhalation. A well-versed understanding of the decomposition process also goes a long way in predicting and preventing any unforeseen explosive or volatile reactions.
Environmental Implications of Decomposition Reactions
While decomposition reactions are instrumental in many beneficial processes, they can also contribute to environmental challenges. For example, decomposing organic waste in landfills releases methane, a potent greenhouse gas. Similarly, certain agricultural practices produce nitrous oxide, another greenhouse gas, when fertilizers decompose. Understanding the environmental implications of these reactions is crucial for developing strategies to mitigate negative impacts, such as capturing and reusing emitted gases or finding alternative materials that decompose more benignly.
The Role of Catalysts in Speeding Up Decomposition
Catalysts are substances that increase the reaction rate without being consumed. In the realm of decomposition reactions, they can significantly speed up the breakdown of compounds. One classic example is the decomposition of hydrogen peroxide. In the presence of manganese dioxide, hydrogen peroxide rapidly decomposes into water and oxygen. By understanding the role of catalysts, researchers can make reactions more efficient and discover potential applications in industries from waste management to energy production.
Historical Insights: Decomposition Reactions in Ancient Times
Our ancestors might not have termed them “decomposition reactions,” but they certainly harnessed their power. Ancient Egyptians used decomposition reactions during mummification, where compounds would break down to preserve bodies. Early metal workers, through trial and error, recognized that heating certain minerals (ores) would decompose them, yielding pure metals. While the mechanisms were not understood then, these practices laid the groundwork for modern chemistry and our comprehensive understanding of decomposition reactions.
Pedagogical Strategies: Teaching Decomposition Reactions
Educators recognize the challenge of making abstract concepts tangible for students. Decomposition reactions, despite their simplicity, can be complex to grasp fully. Innovative teaching strategies include hands-on experiments, like decomposing baking soda using vinegar or employing digital simulations to visualize molecular interactions. By blending theory with practical experiences, educators can instill a deeper appreciation and understanding of decomposition reactions in the minds of budding chemists.
Conclusion
Decomposition reactions, in their elegant simplicity, highlight a fundamental truth about nature: creation and decomposition are two sides of the same coin. Understanding the intricacies of these reactions provides insights into the natural world and paves the way for innovations in various sectors, from healthcare to manufacturing. As science progresses, the potential applications and depth of knowledge surrounding decomposition reactions will expand, making them an ever-fascinating field of study.
Also, Read The Following: Google baseball unblocked










Discussion about this post